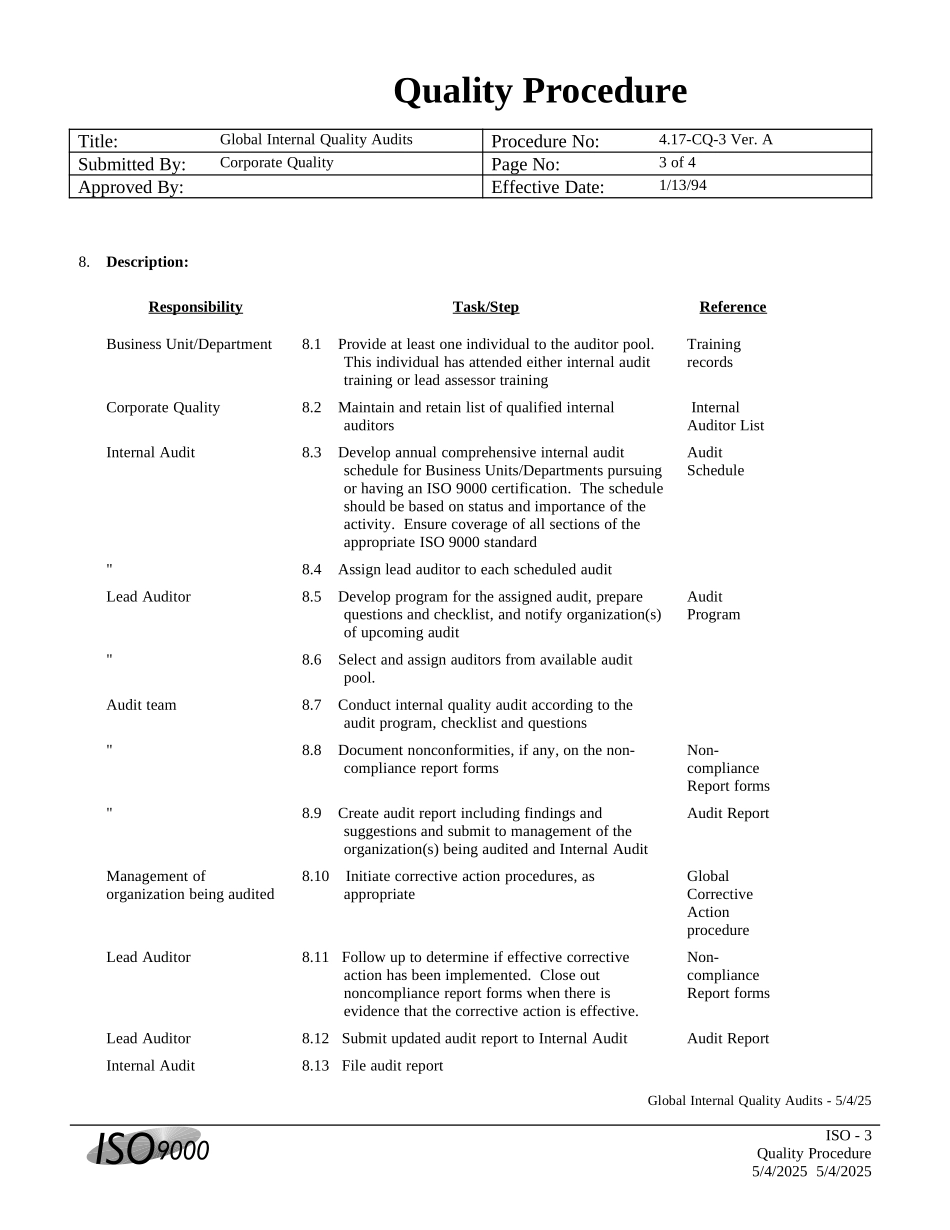
QualityProcedureTitle:GlobalInternalQualityAuditsProcedureNo:4.17-CQ-3Ver.ASubmittedBy:CorporateQualityPageNo:1of4ApprovedBy:EffectiveDate:1/13/941.DOCUMENTHISTORY:RevisionNo:DescriptionofChange:EffectiveDate:AffectedSections:AInitialReleaseofDocument1/13/94AllCopyNo:Issuedto:IssueDate:IssuedBy:ApprovedBy:(Name/Title)GlobalInternalQualityAudits-5/4/25ISO-1QualityProcedure5/4/20255/4/2025QualityProcedureTitle:GlobalInternalQualityAuditsProcedureNo:4.17-CQ-3Ver.ASubmittedBy:CorporateQualityPageNo:2of4ApprovedBy:EffectiveDate:1/13/942.Purpose:2.1Thepurposeofthisprocedureistocarryoutacomprehensivesystemofplannedanddocumentedinternalqualityauditstoverifywhetherqualityactivitiescomplywiththedocumentedqualitysystem.3.Scope:3.1Thisprocedureismandatoryforallpersonnelassignedandauthorizedtoperforminternalqualityaudits.4.ProcedureInterfaces:4.1Inputs:4.1.1None4.2Outputs:4.2.1None5.Referencedocuments:5.1ABCQS4.15.2ABCQS4.25.3ABCQS4.175.4ABC9000QualitySystemPolicyManual6.AuthoritiesandResponsibilities:6.1ChangestothisdocumentmustbeapprovedbytheVicePresidentofCorporateQuality,ordesignee.6.2TheDirectorofInternalAuditisresponsiblefortheimplementationanduseofthisprocedure.7.DefinitionsandTerminology:GlobalInternalQualityAudits-5/4/252-ISOQualityProcedure5/4/20255/4/2025QualityProcedureTitle:GlobalInternalQualityAuditsProcedureNo:4.17-CQ-3Ver.ASubmittedBy:CorporateQualityPageNo:3of4ApprovedBy:EffectiveDate:1/13/948.Description:ResponsibilityTask/StepReferenceBusinessUnit/Department8.1Provideatleastoneindividualtotheauditorpool.ThisindividualhasattendedeitherinternalaudittrainingorleadassessortrainingTrainingrecordsCorporateQuality8.2MaintainandretainlistofqualifiedinternalauditorsInternalAuditorListInternalAudit8.3DevelopannualcomprehensiveinternalauditscheduleforBusinessUnits/DepartmentspursuingorhavinganISO9000certification.Thescheduleshouldbebasedonstatusandimportanceoftheactivity.EnsurecoverageofallsectionsoftheappropriateISO9000standardAuditSchedule"8.4AssignleadauditortoeachscheduledauditLeadAuditor8.5Developprogramfortheassignedaudit,preparequestionsandchecklist,andnotifyorganization(s)ofupcomingauditAuditProgram"8.6Selectandassignauditorsfromavailableauditpool.Auditteam8.7Conductinternalqualityauditaccordingtotheauditprogram,checklistandquestions"8.8Documentnonconformities,ifany,onthenon-compliancereportformsNon-complianceReportforms"8.9Createauditreportincludingfindingsandsuggestionsandsubmittomanagementoftheorganization(s)beingauditedandInternalAuditAuditReportManagementoforganizationbeingaudited8.10Initiatecorrectiveactionprocedures,asappropriateGlobalCorrectiveActionprocedureLeadAuditor8.11Followuptodetermineifeffectivecorrectiveactionhasbeenimplemented.Closeoutnoncompliancereportformswhenthereisevidencethatthecorrectiveactioniseffective.Non-complianceReportformsLeadAuditor8.12SubmitupdatedauditreporttoInternalAuditAuditReportInternalAudit8.13FileauditreportGlobalInternalQualityAudits-5/4/25ISO-3QualityProcedure5/4/20255/4/2025QualityProcedureTitle:GlobalInternalQualityAuditsProcedureNo:4.17-CQ-3Ver.ASubmittedBy:CorporateQualityPageNo:4of4ApprovedBy:EffectiveDate:1/13/94GlobalInternalQualityAudits-5/4/254-ISOQualityProcedure5/4/20255/4/2025QualityProcedureTitle:GlobalInternalQualityAuditsProcedureNo:4.17-CQ-3Ver.ASubmittedBy:CorporateQualityPageNo:5of4ApprovedBy:EffectiveDate:1/13/949.GuidelinesforPerformingProcedure:9.1NoncomplianceReportformsareconfidentialrecordsandwillnotbemadeavailabletoanyoneoutsidethecompany,includingsecond-andthirdpartyauditorsandregulatoryagencies.9.2Auditors,includingtheleadauditor,areselectedonthebasisofavailability,trainingandorganizationalaffiliation.10.ObjectiveEvidence:10.1Auditschedule10.2Auditprogram(s)10.3Non-compliancereportform(s),ifany10.4Auditreport(s)GlobalInternalQualityAudits-5/4/25ISO-5QualityProcedure5/4/20255/4/2025